THE NEXT PHAGE
Popular Science April 2009
By Elizabeth Svoboda Posted 03.31.2009 at 7:11 am
How to heal an infection that defies antibiotics? Another infection. Doctors in Eastern Europe have used lab-grown viruses to safely cure millions of wounds. So why can't we do the same here?
It seemed like nothing at first. The red patch that appeared on Roy Brillon's thigh could have been a spider bite. But as the weeks passed, it grew and grew. By December 2004, the innocuous-looking bump had become an open wound the size of the palm of his hand. Brillon's doctor, Randy Wolcott, prescribed just about every antibiotic he could think of to cure the infection, but the lesion just got worse. "It was really bad," says Brillon, a 62-year-old retired housepainter from Lubbock, Texas. "I had to give up work because I couldn't climb ladders anymore."
Brillon felt like he was being eaten away from the inside out. And in a very real sense, he was. Left unchecked, bacteria like the streptococcus and staphylococcus devour soft tissue to keep themselves alive, leaving ragged red edges that expand outward with terrifying aggression. The pain in his leg was so excruciating that Wolcott prescribed morphine. "I was only supposed to take two pills a day, but I was taking three in the morning and three in the afternoon," he says. "The pain is indescribable. You just grit your teeth."
As head of Lubbock's Southwest Regional Wound Care Center, Wolcott knew well the typical prognosis for patients with antibiotic-resistant infections like Brillon's: gangrene, amputation and, for about 100,000 Americans a year, death. " 'Chronic wound' is a code word for 'you can't heal it,' " he says. "The hallmark is, we cut it off or we cut it out. It's pretty barbaric." Wolcott was desperate for an alternative. After putting in 10-hour days at the clinic, he often sat up late at night poring over medical journals for the newest wound-care research—something, anything that might help patients with the most intractable infections.
When Brillon arrived for a follow-up appointment three weeks later, Wolcott entered the room with a dropper in one hand and a vial of liquid that looked suspiciously like pond water in the other. The liquid, it turned out, was Wolcott's "anything": a murky concoction filled with bacteria-eating viruses known as bacteriophages. Physicians in Eastern Europe, Wolcott had explained to Brillon earlier, have been using phages safely since the 1920s to treat conditions that defy conventional antibiotics, from strep and tuberculosis to infected sores like his. Even U.S. drug companies sold them until the early 1940s, when penicillin came along and proved easier to use, generally more effective and, in the end, more lucrative than phages. The viruses might not help, he admitted, but if they didn't hurt, what was the harm in trying?
Brillon didn't need much convincing. The Food and Drug Administration was another story. Since 1963, the agency has mandated a strict approval process for all medications sold in America. Phage therapy has yet to be subjected to it, so Wolcott had to petition his state regulatory board to allow him to administer it only to people who had exhausted all other options. Then, because you can't find phages in U.S. pharmacies, he had to trek all the way to the former Soviet republic of Georgia to get it. There it's sold over the counter like eyedrops. He bought, for $2 each, three clear glass bottles, each filled with a liquid containing hundreds of types of phages.
"That's it?" Brillon asked, after Wolcott dribbled a few drops of the yellowish liquid onto his wound. The stuff was painless. Nothing much happened over the first few days, and Brillon braced himself for another disappointment. But as the week passed, the sore began to fade to a healthier pink, and then a new island of healthy skin emerged, expanding steadily every day. Within three weeks, the wound was completely healed. "You'd better take pictures of this," Brillon told Wolcott, "or nobody is going to believe it."
BRILLON'S RECOVERY was astonishing, but it wasn't a one-shot deal. Wolcott had also given the phage solution to 10 of his other worst-case patients, and many of them were showing similar results. If phages worked for them, Wolcott reasoned, couldn't they also work for the millions of patients in the U.S. living with infections resistant to antibiotics? His patients, he felt, were proof of it. The real question was whether he could convince the FDA.
As viruses go, phages are relatively benign. They're the most abundant naturally occurring organisms on Earth. They can be found virtually everywhere-in soil, drinking water, sewage. In fact, each one of us naturally has billions of them in our bodies. They prey only on bacteria, never human cells, they rarely spread from person to person, and, perhaps most important, bacteria have trouble becoming immune to them. As living organisms, phages are constantly changing and adapting in tandem with their host bacteria to kill them more effectively. Phage therapy could therefore eliminate the vicious cycle in which bacteria evolve resistance to antibiotics, necessitating the development of new, even more powerful drugs, at which point the process begins all over again.
But there's a big sticking point. The very characteristic that makes phages so effective-that expert ability to shape-shift- makes it difficult for them to pass muster with U.S. regulatory authorities. Although there have been no reports of adverse effects resulting from mutations, phages that don't normally nest inside the human body could potentially swap genes with other phages that do and produce foreign proteins that trigger an immune reaction. And it's impossible to say exactly how a virus might mutate when exposed to different bacteria, says Paul Sullam, a microbiologist at the University of California at San Francisco. For that reason, among others, says FDA spokesperson Karen Riley, phage therapy used to treat or cure humans must be regulated as a biological product. That means that if the viruses show serious signs of mutating or changing during clinical trials, even if those changes pose no risk to patients, the trials could be scrapped. Which explains why Big Pharma isn't eager to conduct them. "I understand where the FDA is coming from, because each phage poses a certain risk," Sullam says. "When viruses have the ability to exchange genetic material, it makes people nervous on a visceral level."
TO THE FDA, the serum Randy Wolcott drizzled on his patient's leg is new and unproven. But to the scientists working at the George Eliava Institute of Bacteriophage, Microbiology and Virology in the republic of Georgia, the medicine is as trusted as aspirin. Since 1923, when the facility was founded, scientists there have successfully treated millions of patients with phage therapy and presented more than 100 research abstracts at international conferences attesting to its clinical value.
Wolcott calls Eliava the "mother ship of phage research," a worldwide Mecca for people suffering from antibioticresistant infections. Only it doesn't look like the sort of place you'd want to go with a health problem. When Wolcott visited to hunt down alternatives for his patients, the four-story facility bore a closer resemblance to a neglected sanatorium. The walls were unpainted, the rooms were dark, and the equipment looked like museum pieces. "The conditions were abysmal," he says. "Yet the science is amazing."
Wolcott spent five days shadowing the staff and learning about phage therapy firsthand. "They had these 10-foot-tall fermenters, like big cooking pots, and they used them to make millions of doses of phage medication a year," he says. What surprised him most, aside from the dreary decor, was the painstaking way each prescription was custom-tailored for the patient. Phages are species-specific-different strains attack different bacteria. Since some wounds can harbor hundreds of different types of bacteria, physicians there first culture a tissue sample of the infection to determine its precise bacterial composition. The next step is to brew a custom cocktail of sometimes hundreds of phages selected from the institute's vast library of thousands. This whole process can take up to four days. The treatment-often administered through an IV bag that drips phage liquid directly into patients' wounds for 24 hours a day-can last up to two weeks.
As inconvenient as the procedure sounds, few people complain about it. The results are spectacular, Wolcott says: "I met a woman with a chronic ear infection who was coming back to the phage clinic for her final appointment. They gave her the therapy, and within a week, she was completely cleared up." In fact, studies published over the past several decades, based on trials conducted at Eliava and elsewhere in Eastern Europe, have shown that phage therapy has an 80 to 90 percent success rate against bacteria likely to show antibiotic resistance, such as Staphylococcus aureus and Escherichia coli. In contrast, many antibiotics fail outright against the evolved forms of these pathogens. In June 2005, a bacterial strain resistant to the first-line antibiotic imipenem ravaged more than 50 patients at New York City hospitals. Among patients whose infections infiltrated their bloodstream, the death rate was 47 percent.
With antibiotic resistance reaching record levels worldwide, phage therapy is no longer the sole province of Eastern European researchers. British biotech firm Biocontrol wrapped up the first Phase II clinical trial of phages in Western Europe last year with dramatic results. Its phage regimen combats the Pseudomonas aeruginosa bacterium, which causes serious lung and ear infections and is highly resistant to antibiotics. Patients with antibiotic-resistant infections who received phage therapy experienced a 50 percent reduction in their symptoms, compared with only a 20 percent decrease in the group that did not receive phages. "Frankly, I was blown away," says Dan Nelson, a biochemist at the University of Maryland who was at the conference where Biocontrol unveiled its results.
For Wolcott, who watches hundreds of patients die every year from seemingly incurable infections, these medicinal viruses can't arrive in the U.S. fast enough. "Phage needs to be fast-tracked. It works. It's completely natural. Why can't you spray this stuff on a kid's throat right now?"
IN TRUTH, phage therapy is already here, just not in a way that's practical for Wolcott. Owing to a regulatory technicality, manufacturers can use phages to keep ready-to-eat foods like deli meat and coleslaw safe from bacterial contamination, but doctors can't prescribe them to treat a bad case of strep. The difference, according to the FDA, is the application: Spraying a phage on lunch meat makes it a food additive. Give it to someone with an infection, and it becomes a drug. In 2006, the biotechnology company Intralytix took advantage of the less stringent regulatory rules for food additives to secure FDA clearance to sell a phage spray that kills Listeria bacteria, a common source of food poisoning. The company is also developing several phage treatments for humans, one of which entered clinical trials, thanks to Wolcott.
Wolcott met Intralytix CEO John Vazzana at a phage conference in Texas three months after he returned from Europe. The two talked for hours about the frustrating plight of phages in the U.S. By the end of the conference, they had hatched a plan to convince the FDA to let them start a clinical trial. Intralytix would supply the phages; Wolcott, the patients.
The FDA eventually agreed, on the condition that they limit the trial to only eight well-studied Intralytix phage strains. Wolcott quickly recruited 39 patients, all with infected venous leg ulcers, and set about conducting a two-year trial, the first of its kind on this side of the Atlantic.
Every week, Wolcott's study participants arrived at the clinic and received their phages through a handheld ultrasonic device, a high-tech upgrade to the IV drips common in Eastern Europe. The device simultaneously sprays on saline-based phage solution and destroys blackened or dead tissue, allowing the phages to penetrate deeper into the wound. Like all initial clinical trials, Wolcott's was designed to assess the safety of the therapy, not its outright effectiveness. In this context, the study yielded promising signs for the future. None of the patients in the trial reported severe side effects, but the efficacy was unimpressive. Nearly 70 percent of the volunteers experienced significant healing by the end of the 24-week trial period, as did a similar percentage of trial patients who did not receive the phage. Wolcott anticipated this, and attributes it to the fact that the cocktail was not tailored to combat the particular bacteria in his patients' wounds, as is standard practice in Georgia, limiting the phages' potency.
And this is where Wolcott hits a wall. FDA regulators have told him that if he wants to use phages on his patients, he's going to have to carry out a separate clinical trial for each phage or particular mix of phages he hopes to administer, just as he would if he were shepherding distinct chemical compounds through the regulatory process. But since each of his patients' wounds might contain hundreds of different species of bacteria, he can't reasonably attempt to conduct trials of the thousands of phage combinations required to combat them all, especially considering that the cost of a trial for a single drug can easily run into the millions. "People in this country have a right to be incensed that we have a very different situation here than in Europe with regards to phage," says Betty Kutter, a phage researcher at Evergreen State College. "Our whole regulatory environment has been one major thing that has slowed people down."
A Phase II efficacy trial enrolling 100 to 200 wound patients would cost about $9 million-and if the therapy being tested included only a few types of phages, as the first trial did, there's a good chance it wouldn't pan out for patients whose infections are caused by multiple strains of bacteria. And given the current regulations, Vazzana isn't sure he could even find the capital to fund the trial.
But phage researchers like Ben Burrowes of the Texas Tech University Health Sciences Center are optimistic. "Companies will start coming out with phage products at some point," he says, "and once those first few get through the approval process, the FDA will relax its standards a little."
RockefelleR university biologist Vincent Fischetti, for one, isn't holding his breath. Fischetti has no doubt that there's a gaping hole in the health-care landscape where effective antibacterial drugs should be. He just isn't sure phages are the best way to fill the void. To him, Wolcott and his fellow phage-therapy practitioners are like peacekeepers with no governmental backing: wellintentioned, to be sure, but unlikely to have much success in the end. "I'm not working on phage therapy," he insists, as he guides me through his sixth-floor lab overlooking the institute's Manhattan campus. "I'm working on phage-based therapy."
This distinction might seem arcane to nonbiologists, but in Fischetti's mind, it's a crucial one. While Wolcott sees phages as a major therapeutic coup, Fischetti sees them as merely an intermediate step toward a new generation of even better bacteria-fighters. He contends that the uphill regulatory battle phages face, as well as the risk of mutations, make them too big a gamble for American drug companies. "Phages are going to be a boutique treatment, nothing more," he says.
So he is taking an alternative approach, purifying the phage to extract the lysin, the enzyme it uses to dissolve the bacterial cell wall and kill the bacterium. He enlists his lab staff to serve as biological prospectors, collecting the bacteria-killing viruses from swamps, rivers, anywhere they can find them. He points to a bag of smelly bat excrement on his windowsill. "We can take the phages out of that stuff."
Having observed that lysins were the phages' "active ingredients," Fischetti aims to harvest the lysins from them and turn them into stable antibacterial drugs. If successful, he could accomplish a double feat previously thought impossible: getting the bacteria-fighting benefits of phages to patients, while doing an end run around the regulatory Rube Goldberg machine that researchers like Wolcott face.
Whereas phages must evolve to keep up with bacterial evolution, lysins are like a blunt instrument that can kill entire families of bacteria, eliminating the need to isolate and test thousands of different compounds as phage scientists in Georgia do. But even if the FDA might perceive lysins more favorably than phages alone, Kutter says, the enzymes have drawbacks of their own. Lysins work only on Gram-positive bacteria, like strep and staph, not Gram-negative bacteria like E. coli and salmonella-the Gram-negative bacteria have an outside membrane that the lysins can't get through. Phages, on the other hand, can work against both kinds of bacteria. And unlike traditional phage therapy, lytic enzymes haven't made it to clinical trials yet, so although petri-dish evidence is promising, there's no telling whether it will translate into success in a hospital setting.
It's clear that unless the FDA is willing to consider revised approval guidelines, phage therapy in the U.S. will remain in a holding pattern indefinitely. And in Lubbock, Roy Brillon's life has settled into a similar stasis. The phage Wolcott brought back from Georgia healed his leg wound, but since Brillon has problems with the valves that control blood flow in his leg veins, he's constantly developing new sores. "The blood goes down the veins, but it can't go back up," he explains. "The sores shrink and then pop up somewhere else on my leg."
What Brillon really needs, Wolcott says, is a phage cocktail custom-mixed to target the particular bacteria colonizing his wounds. But it's back to the same old antibiotics for now. Sometimes, if the bacteria happen to be non-drug-resistant, the antibiotics work; sometimes they yield about the same results as a newt's-eye potion. Brillon keeps his legs wrapped in Ace bandages like a burn victim. "If you don't keep 'em tight," he says, "then the legs swell, and that's when the sores come out."
But he's not dwelling on the obstacles. Instead he imagines the day when he'll be able to put his infections behind him for good and spend more time fishing and playing with his four grandchildren who live nearby. He figures that if anyone can get him there, it will be Wolcott and his army of phages. "It really bothers him if he gets ahold of something he can't handle," Brillon says. "He tells me, 'I'm never going to give up on you.' "
Elizabeth Svoboda is a contributing editor at Popular Science. Her last feature article, in July 2007, was about research efforts to turn algae into clean-burning biodiesel.
Copyright Bonnier Corporation Apr 2009


FDA Friendly?: Bacteria-killing enzymes may help turn phages [like the one above] into stable antibiotics, which could earn them an OK from the Food and Drug Administration
courtesy Vincent A. Fischetti/Laboratory of Bacterial Pathogenesis and Immunology, Rockefeller University
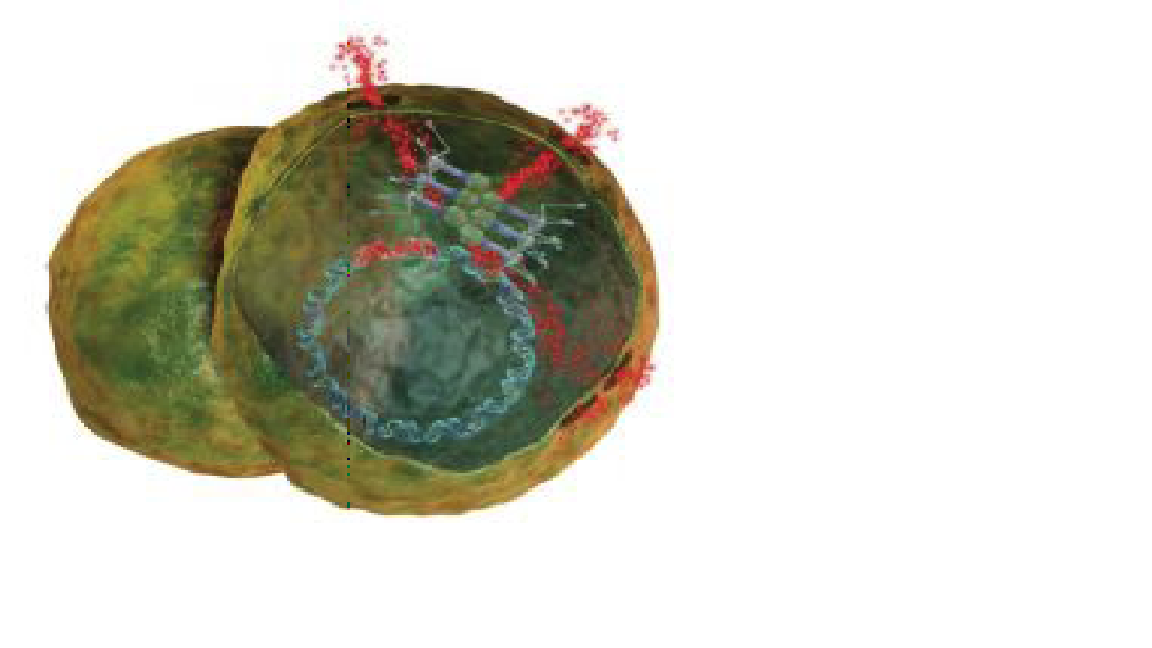
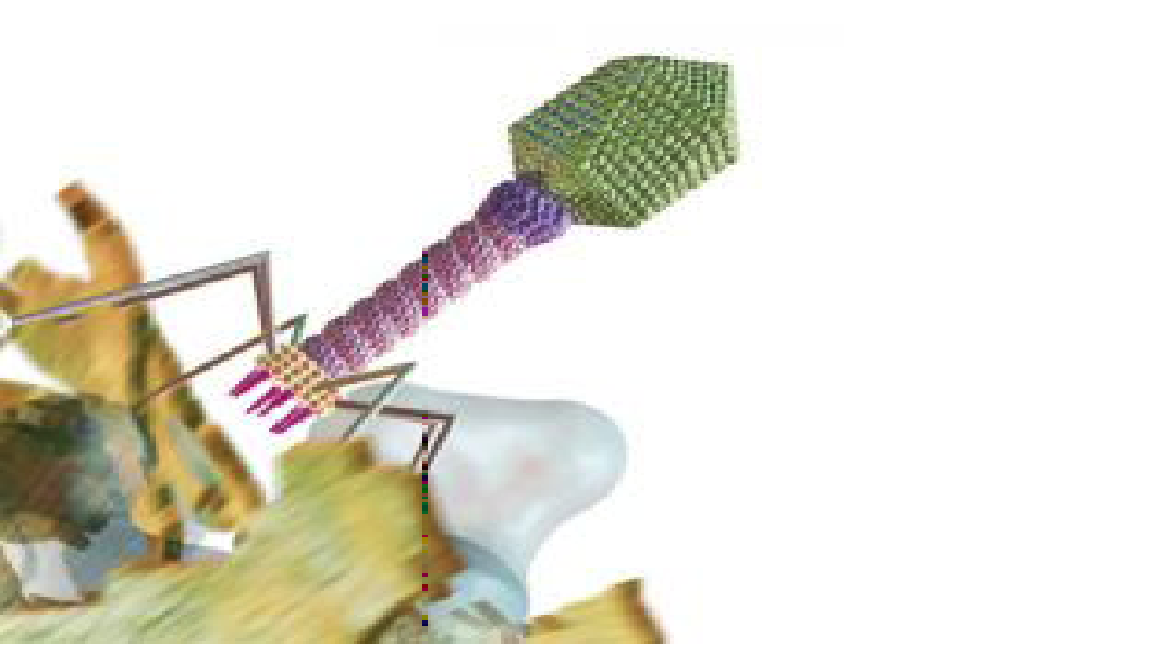
Phage Replication: Once inside the wound, viruses hijack cells and produce enzymes that poke holes in the cell wall Medimation

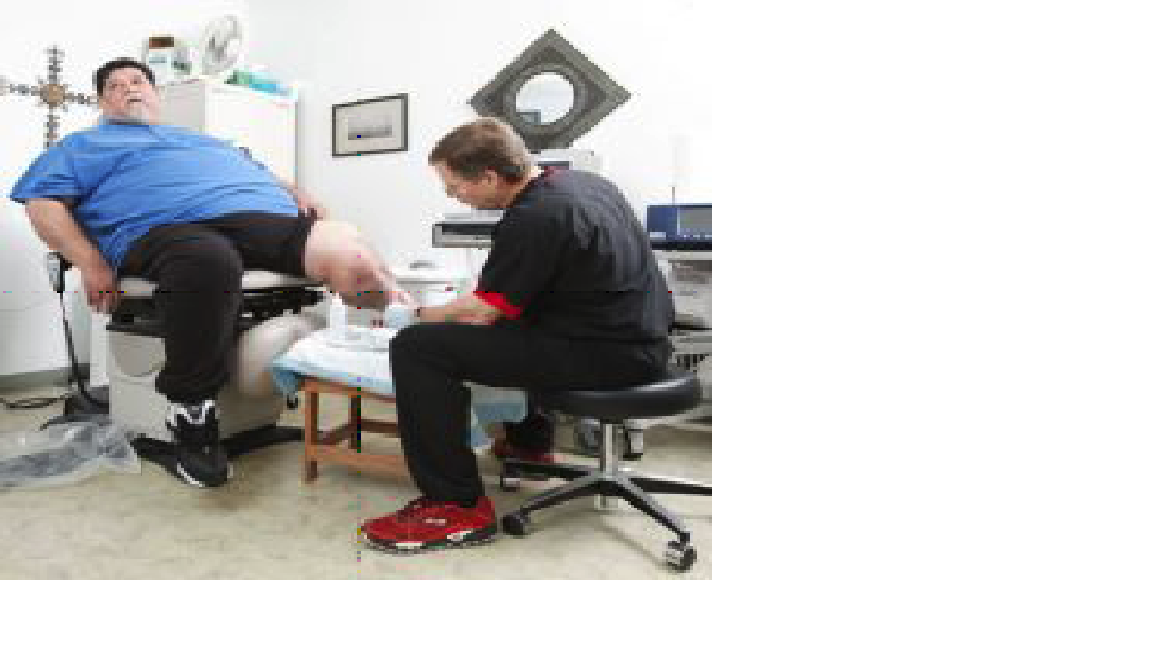
Last Hope: Wolcott [above] has secured federal clearance to administer phages [below] to patients whose wounds defy conventional antibiotics John B. Carnett
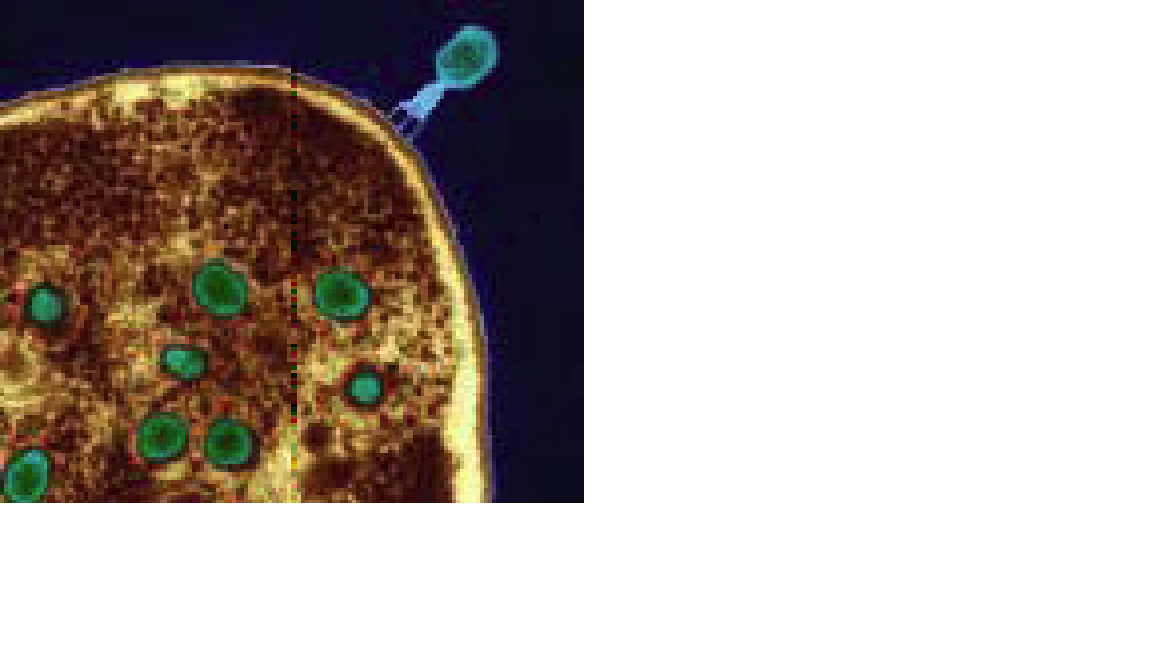
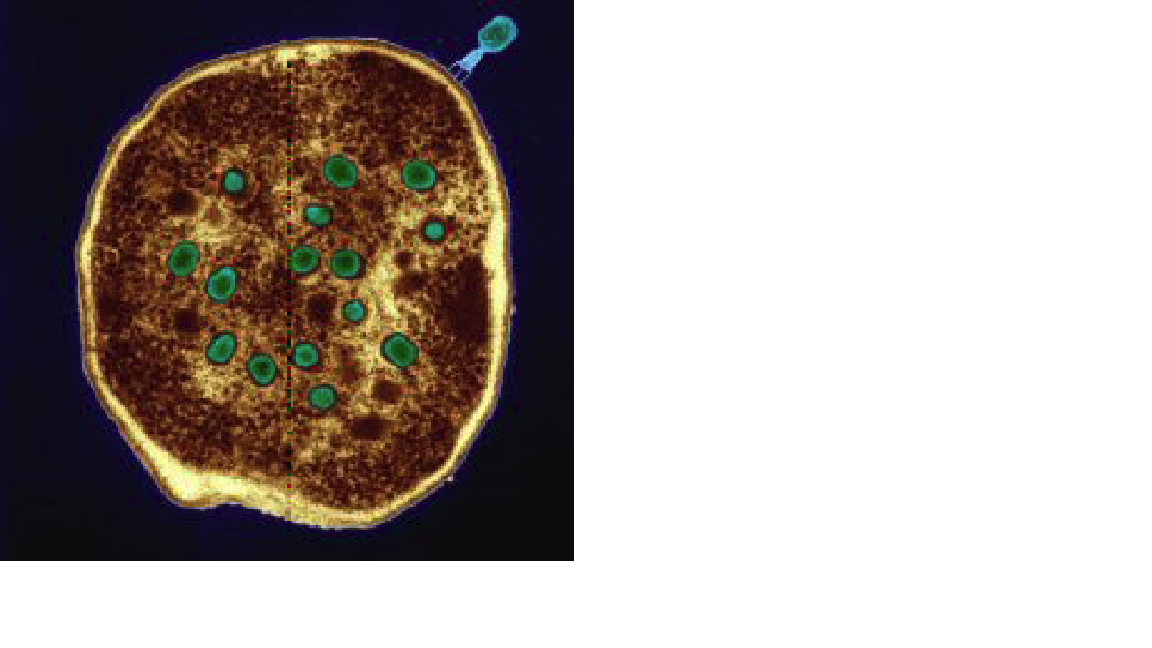
From Georgia with Research: Phages [in green] are as trusted as aspirin in the former Soviet republic of Georgia Eye of Science/Photo Researchers
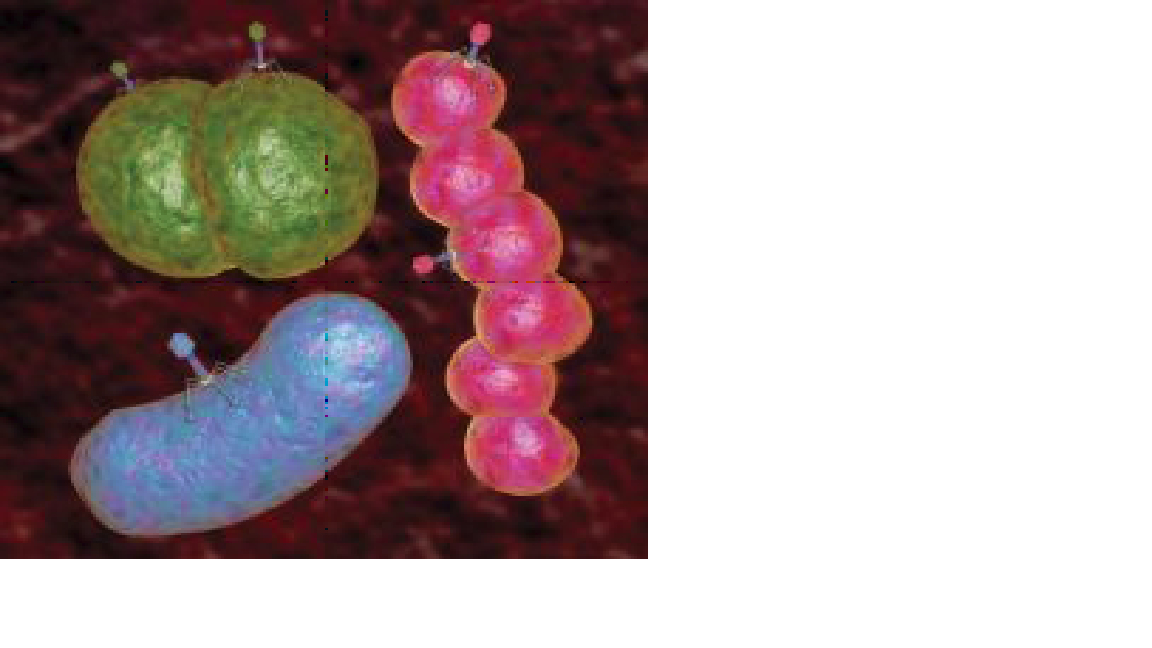
Inside the Wound: The first step in phage therapy is determining the composition of a bacterial colony like this one Medi-mation

Unphaged: Rockefeller University researcher Vincent Fischetti wants to use phages as a basis for an FDAcompliant therapy courtesy Vincent A. Fischetti/ Laboratory of Bacterial Pathogenesis and Immunology, Rockefeller University

Puncturing Bacteria to Cure a Wound: Once they’ve hijacked a bacterial cell, viruses replicate inside it, producing enzymes that poke holes in the cell wall to kill the bacterium and release more infectious phages Medi-mation