W.W.C. Topley and the “Missing” Phage Reference Bacteriophage Ecology Group News
@ www.phage.org by Stephen T. Abedon (editor) December 31, 2007 issue (#26) Paul Hyman & Stephen T. Abedon B Ecology Group News #26William Whiteman Carlton Topley (January 19, 1886 – January 21, 1944) was a noted British epidemiologist and bacteriologist. Educated at the City of London School, St. John’s College, Cambridge and St. Thomas’ Hospital, he received his MD degree and was admitted as a member to the Royal College of Physicians in 1911. He then served as the Director of the Pathological Department at Charing Cross Hospital from 1911-1922. Topley left Charing Cross Hospital to become a Professor of Bacteriology at the University of Manchester, a position he held until 1927 when he was appointed the first Professor of Bacteriology and Immunology at the London School of Hygiene and Tropical Medicine at the University of London.Topley’s research focused on experimental epidemiology particularly of microbial diseases. In 1929 he coauthored, with Graham Wilson, The Principles of Bacteriology and Immunity which is still published as Topley and Wilson's Microbiology and Microbial Infections, currently in its tenth edition. Earlier, while still at the University of Manchester, Topley and his colleagues did work on what was then referred to as the “Twort-D’Herelle phenomenon”. Today we would say he was working with bacteriophages. This work produced two papers as well as a presentation to the Manchester Literary and Philosophical Society. The presentation was made on March 17, 1925, and was subsequently published later that year in the Memoirs of the Manchester Literary and Philosophical Society (also called Manchester Memoirs), a transcription of which begins on the following page. Oddly, it is not listed in the Raettig compendium of bacteriophage publications (see http://www.phage.org/bgnws003.htm#submissions).This talk represents one of the earliest published reviews of the state of bacteriophage research. It was made while the nature of the bacteriophage as a type of organism was still being debated. But the basic culture techniques, isolation, and enumeration methods described are still in use today. We hope that this look back will remind our fellow phage biologists how far we have and have not traveled in our understanding bacteriophage biology.Acknowledgements:We would like to thank the Manchester Literary and Philosophical Society for generously allowing us to reprint Dr. Topley’s article. We would also like to thank Kathy Zabak for proofreading help.Sources:1. William Whiteman Carlton Topley. Wikipedia. http://en.wikipedia.org/wiki/William_Whiteman_Carlton_Topley (accessed December 18, 2007).2. M. Greenwood, William Whiteman Carlton Topley, 1886-1944. Obituary Notices of Fellows of the Royal Society 4(13):698-712, Nov. 1944.3. London School of Hygiene and Tropical Medicine. Chronology. http://www.lshtm.ac.uk/library/archives/chronology.html (accessed December 18, 2007).4. Proceedings of the Manchester Literary and Philosophical Society, Vol lxix, p. xix, 1925.Manchester Memoirs, Vol. lxix. (1924-1925), No. 8.[*]VIII. “The Bacteriophage Phenomenon: Transmissible Bacterial Lysis.”Prof. W. W. C. Topley, M.A., M.D., M.Sc., F.R.C.P., M.R.C.S.In choosing a subject for this paper I have tried to select some bacteriological phenomenon which is of general interest, and I think it is true that the problem I propose to discuss has this general character. It has the additional advantage that it belongs to that interesting category of questions concerning which we are in possession of a considerable mass of well-authenticated facts, but the answer to which seems the more unattainable the more we learn. Transmissible bacterial lysis, indeed, is one of those curious phenomena which obstinately refuse to fit comfortably into our general scheme. The difficulty is not so much that we do not know the facts, as that the facts themselves look all wrong. Since, as I have said, the problem is at least as interesting to the general biologist as to the bacteriologist, involving as it does the consideration of the limit which divides living from non-living things, I hope that some of those whose work has lain along biological, and especially along bio-chemical lines, will have some suggestions to offer.Historically the facts are these. In 1915 Twort described a curious appearance in cultures of a micrococcus, which he had grown from contaminated vaccine lymph. Scattered among the confluent bacterial growth on a culture on ordinary solid nutrient agar, he found glassy areas, which, when examined microscopically, appeared to consist of a finely granular material. These vitreous areas tended to extend over the greater part of the surface growth, converting all the cocci into the granular material. If one of the vitreous areas was touched with a platinum wire, and a normal culture of the coccus was inoculated in one spot with a tiny particle of the granular material, a similar glassy area appeared, which again tended to spread throughout the surface-growth. A suspension of the granular material could be filtered through a porcelain candle, which held back all micro-organisms, but gave a filtrate, a drop of which would initiate the process on a new series of cultures. Such filtrates retained their activity over many months. They withstood heating for one hour at a temperature of 52°C., but not at 60°C. They were most active against young and actively growing organisms. They had no action on dead organisms. The lytic substance, whatever be its nature, could be transferred in an indefinitely long series from culture to culture of the bacteria on which it acted. It could not be cultivated on any known medium, apart from the living bacteria on which it acted. It acted on certain cocci nearly related to the species from which it was obtained, but it was inactive against unrelated bacteria of various kinds.D’Herelle, in 1917, described, quite independently, a phenomenon which he observed in examining the action of filtrates of cultures, from the excreta of dysenteric patients, on the dysentery bacilli isolated from these cases. As will be seen, the observations which he records bear a striking resemblance to those reported by Twort, and almost all subsequent workers are agreed that the phenomena are essentially similar, though showing minor differences. D’Herelle himself, however, has strenuously denied this, and regards his lytic principle, which he asserts to be a living micro-organism, a parasite of the bacterium, as entirely distinct from Twort’s lytic substance. This supposed ultramicroscopic parasite D’Herelle has named the Bacteriophage.Briefly summarized, D’Herelle’s most important demonstrations have been as follows: Filtrates from the excreta of typhoid and dystentery [sic] convalescents contain, in a large proportion of cases, a lytic principle which has the power of dissolving or inhibiting the growth of typhoid or dysentery bacilli, and which can be transmitted in series indefinitely. This action may be shown by adding a drop of lytic filtrate to a young broth culture of a sensitive organism, which shows subsequent partial or complete clearing.Such filtrates are usually not strictly specific in their action—that is, they are usually active against certain closely related bacteria, as well as against the actual bacterium infecting the patient from whom the filtrate was obtained.The activity of the lytic principle is often feeble when first isolated, but it rapidly increases when transmitted in series from one culture of the sensitive organism to another, so that eventually a few drops of a filtrate diluted 10,000,000 times or more will suffice to initiate the lytic phenomenon. At the same time it frequently happens that the range of activity, as regards the microbial species acted upon, extends with the absolute increase in lytic power. So that the later filtrates of a series will produce lysis of bacterial species which before were unaffected. In some cases, if a given lysin is transmitted in series through cultures of a bacterium other than that from which it was originally obtained, it gains the power of lysing this heterologous organism in very high dilutions, while leaving unaffected, or even losing more or less completely, its power to lyse the original homologous bacterium. Thus, the lytic agent appears to show in some degree the power of adaptation.Lysis occurs most readily in young, actively growing cultures of the sensitive bacterium. Antiseptics or temperatures, which in any way retard the growth of the bacteria without killing them, interfere with the lytic principle, though they neither destroy it nor lessen its activity, when it is transferred to fresh cultures.It is impossible to transmit the lytic principle in dead cultures of bacteria or in bacterial filtrates. The presence of living multiplying bacteria is essential.There is evidence that the lytic principle is particulate in nature. If the action of a lytic filtrate be studied by allowing a drop of such a filtrate, in increasing dilution, to run over the surface of an agar slope thickly inoculated with a susceptible bacterium, the lytic action, in suitable dilutions, is evidenced by the appearance of isolated circular clear areas, with sharply circumscribed borders. These clear circular areas the plaques vierges of D’Herelle and the subsequent French workers, the löcher of the German investigators, are regarded by D’Herelle as colonies of the bacteriophage which have dissolved the surrounding bacteria. He has recorded experiments, in which the use of progressive dilutions of a lytic filtrate has allowed these colonies to be counted, in much the same way as we count bacteria in a fluid culture. A filtrate giving 200 lytic areas at a given dilution will give 20 such areas when the dilution is increased tenfold, and so on. He has also demonstrated that prolonged centrifugalisation increases the concentration of the lytic principle in the lower layers of the fluid, so that the count of the lytic areas is increased.In the course of pathological work with which this paper is concerned, I have had occasion to observe many of the phenomena referred to above. To obtain a clear picture of the fundamental facts, we may consider the technique employed in isolating and testing a lytic filtrate. It is not necessary to have recourse to a man or animal suffering from any particular infection, for, whatever may be our view about the nature of the lytic principle, there is now no doubt that a substance, or substances, capable of initiating lysis in many species of susceptible bacteria are commonly present in the human or animal intestinal canal.We start, then, by taking a specimen of intestinal content and transferring it to an adequate quantity, say 250c.c., of ordinary nutrient broth. We incubate this for 24 hours or more at 37°C. We act here on the assumption that, if the sample of intestinal contents selected contains a lytic principle, it will contain bacteria on which that lysin acts. If this assumption be correct there will occur, during the period of incubation, multiplication of sensitive bacteria in the presence of the lysin, and this will lead to an increase in the concentration of the lysin itself. At the end of 24 hours, or after some convenient period, we filter the mixed bacterial culture through a porcelain candle of suitable porosity, a Chamberland L2 is the type usually employed. This will give a bacteria-free filtrate which may or may not contain a lysin, active against the bacterium which we have selected for study. In choosing our bacterium, if our desire be simply to study some problem in connection with bacteriophage action in general, we shall select some species which is known to be sensitive to lysins frequently found in normal animal excreta. The B. dysneteriæ of Shiga is a good example. We shall now inoculate a flask of broth or of peptone-water with the bacterium selected, and incubate it for a few hours to allow of a moderate degree of bacterial growth. We shall then add a few c.c. of our filtrate and return the flask to the incubator. Lytic action may be obvious within a few hours, as shown by a partial or complete clearing of the culture, followed in many cases by a subsequent increase in turbidity due to secondary bacterial growth. If, however, there is no such obvious evidence of lysis, we shall take a loopful of the culture and inoculate a tube or plate of solid medium, so as to allow of copious surface growth. We shall examine these cultures after a further 24 hours’ incubation, and may find that the growths show the presence of typical plaques vierges, or of the curious malformed, bitten or nibbled colonies, which are scarcely less characteristic of bacteriophage action. If, at this stage, we find that a lytic agent is present, we shall proceed to increase its activity by a series of transmissions, or passages, in cultures of the sensitive organism. For this purpose we shall filter our first lytic culture, and add some drops of the filtrate to a second young and actively growing culture of the sensitive organism. This process we shall repeat again and again, finally obtaining a filtrate which may cause complete clearing of young broth cultures of our sensitive bacterium in a few hours. If we wish to obtain a quantitative measurement of our final product, we proceed by the method of dilution, testing the action of constant amounts of progressive dilutions of our filtrate on the surface growth of cultures of our susceptible bacterium. If the lytic areas are well marked, we may actually enumerate them, and make a count of the number of lytic particles in our original filtrate. If the lytic areas are ill-defined we may simply test progressive dilutions until we find the limit at which evidence of bacteriophage action ceases. It has become usual to proceed by progressive tenfold dilutions, and to express the activity of a filtrate by means of a lysin exponent, which is simply the index expressing the power of 10 which corresponds to the highest dilution which yields evidence of lytic action—this is, the lysin exponent is simply the logarithm of the highest active dilution. It is by no means unusual to obtain lysin exponents of nine or more—that is to say, filtrates diluted at least 1,000,000,000 times may still be active.The original observations of Twort and of D’Herelle have been followed by a vast amount of research work during the past eight years, represented by a terrifying mass literature. It is quite impossible to do more than indicate briefly the more important of the additional facts which have come to light, and the rival theories which have been based upon them. It may be said at once that D’Herelle’s conception of the lytic principle as a living ultra-microscopic organism has not gone unchallenged. It has, indeed, met with a rather limited acceptance, and at one period had few supporters. It is still true to say that the general trend of opinion is against this explanation, but the difficulties presented by other interpretations have produced a slight reaction in favour of D’Herelle’s view.The opposing theories are three in number. The lytic principle has been regarded as a ferment, which produces some change in the bacteria in consequence of which they not only undergo lysis, but themselves produce the same ferment, or some similar one, which in its turn acts on successive bacterial generations. In support of this conception experiments are recorded which show that the lytic principle has, in fact, many of the properties of a ferment. Its heat resistance, which is higher than Twort believed, and is certainly not less than 70°C., corresponds well with the know resistance of any ferments. The lytic principle can be precipitated with actone [sic] or with an ether-alcohol mixture, and can be recovered almost intact. It can be absorbed by colloidal metals. It can be removed from a filtrate by absorption with aluminum-hydroxide, and recovered by solution in acetic acid. The degree to which it is retained in the pores of a candle varies with the hydrogen-ion concentration of the filtered fluid. If the hydrogen-ion concentration be increased more of the lytic principle is retained during filtration; if it be diminished more passes through. The lysin can be absorbed with Kuselgur, and recovered by solution in weak ammonia.Another conception, that of Bordet and his school, is somewhat more vague. It is the theory of heredity transmissible autolysis. It is enunciated by Bordet himself as fellows: “Under some disturbing influence, the nature of which we remain quite free to discuss, a nutritive vitiation of the bacterium is primarily induced testified to by the appearance of the lytic agent. After this the interference of the external agent is no longer necessary. Henceforth, the reproduction of the principle requires nothing more than the presence of living microbes, which, having absorbed a sufficient quantity of it, liberate new amounts of the same again at a certain stage of their evolution.”Bordet bases his argument largely on the proved necessity for the participation of actively multiplying bacteria in the process of increasing the activity of the lytic principle. It is not enough that the bacteria should be alive, they must be multiplying in order that a lytic substance should be set free. Bordet’s hypothesis can certainly be reconciled with many of the known facts, and deserves the careful consideration which should be accorded to all his views, but the essential nature of the transmissible autolysis remains to be explained.A third view is that the lytic principle, the bacteriophage itself, represents some stage in the life-history of the parasite, or some alternative if abnormal mode of reproduction, so that once the appropriate stimulus is provided bacterial multiplication is given a new direction, in a proportion at least of the bacteria attacked.One point which has been fully established, especially perhaps by Arkwright, is that the lytic principle is a potent cause of bacterial variation. Under its influence abnormal forms appear. Many of these are resistant to the action of the lysin, and attempts to propagate it in cultures of such variant strains result in failure. Some variants give rise to large mucoid colonies, quite unlike those of the original strain from which they were derived. These sometimes remain mucoid indefinitely in subsequent generations, sometimes the lytic principle must be frequently reapplied in order to maintain the propagation of this type. Sometimes the variants are non-motile, when the original bacterium was motile. Some times they are unaffected by certain antisera which agglutinated the original strain from which they came.The supporters of these latter hypotheses are alike in believing that, after the original stimulus, the lytic principle is elaborated by the bacteria themselves, and support has been sought for this view by attempts to show that the lysin may be found in old cultures of pure bacterial strains, which have been cultivated in laboratories for many years, and which have not therefore been subject to contamination with the supposed ultra-microscopic bacteriophage, which D’Herelle believed to be a normal inhabitant of the intestinal tract of most animal species. In spite of repeated attempts, it may be said with some confidence that it is only very occasionally that the lysin can be demonstrated in old cultures, which have been long isolated from their natural surroundings, and hence from all chance of contamination. Since we know that the lytic principle may be transmitted through many successive sub-cultures, without its presence being obvious, unless special efforts are made to demonstrate it, the supporters of the theory of the ultra-microscopic parasite may fairly contend that such occasional successes are to be expected.It is, perhaps, well to emphasise that, although the lysin has many of the properties of a ferment, it does not behave in the same way as the ferments most familiar to the biochemist. There is no evidence that the bacteria are digested, that there is any protein cleavage in the chemical sense. The phenomenon is that of cytolysis, the disintegration of the bacterial cell, as such, and the setting free of its protoplasmic contents. It is interesting in this connection to note that the presence of colloids in the fluid medium such as gelatine for example, prevents the occurrence of the lytic phenomenon.It is perhaps natural to enquire whether a morphological study might not assist in elucidating the problem. Unfortunately, here, as in the study of undoubted filterable viruses, we are at the limit of microscopical observation. We can detect the changes in the bacteria themselves. Under the action of a lytic filtrate they increase in size, often to an enormous extent, and may assume bizarre shapes. They tend to become clumped together in masses. Their protoplasm, at first clear, becomes opalescent and then definitely granular. Many of these granules are quite clearly observable with dark-ground illuminations, but their exact morphology cannot be made out. A broth culture which has undergone lysis may show nothing but these granules. A preparation made from a colony on solid media, showing lytic change, will frequently show, when observed by dark-ground illuminations, normal or distorted bacteria and bacterial ghosts, lying among masses of this granular material.It is, I think, true that the morphological study of this phenomenon has been somewhat neglected, in comparison with the attention which has been directed to its study by other means, and we may hope that better methods of microscopy, and especially microphotography, may yield information of real value. It is, however, probable that we shall have to wait for technical improvements. The limits of resolution imposed by the wave-lengths of the light used for illumination form, at the moment, an insuperable barrier to the adequate study of the real form of particles of this order of size.I do not want to deal at any length with the pathological or medical aspects of the problem, but as they may be regarded as yielding some relevant data concerning its essential nature, they may be touched on briefly. It is clear that, if the bacteriophage be in reality a parasite of a parasite, a being which attacks and kills bacteria, it is a conceivable hypothesis that the administration of a lytic filtrate, active against bacterium A, to animal B, in whose tissues bacterium A is multiplying with disastrous results, will terminate the conflict in B’s favour. On this point D’Herelle himself has no doubts. The discovery of the bacteriophage has, for him, revolutionized the study of immunity, and rendered our present-day conceptions antiquated and useless. He believes that infective diseases can be cured by the administration of an appropriate bacteriophage culture, and that the whole story of epidemiology is simply a history of the conflict between bacterial parasites and their still smaller foes, with animals or man as interested but relatively quiescent spectators. He believes that it is just as possible to catch an attack of immunity against a disease, as to catch the disease itself—a conception, by the way, that has many supporters, quite apart from the factor of bacteriophage lysis. He considers that it should be possible to stop an epidemic of typhoid, for instance, by adding a suitable bacteriophage to the public water supply of the affected town. It would be unwise to predict the final verdict as to the importance of bacteriophage lysis in the treatment or prevention of disease, but it is fair to say that skeptics exist. In my own Department, since we are particularly interested in the experimental study of epidemic disease, we have submitted this question to direct experiment. It is sufficient to say that, up to the present, our results have not led us to adopt an optimistic attitude. From the point of view of preventing or cutting short an experimental bacterial infection, the bacteriophage would appear to be a singularly inert substance.We may consider one point more. D’Herelle has adopted quite definitely, the theory of the big flea and the little flea, and adds “and so ad infinitum” to his creed without a qualm. He states quite clearly that we have no right to set a lower limit to the side [sic] of living organisms. This is, of course, absurd. It is quite easy to set a lower limit to the size of living organisms, if we mean by that term living cells. We know that there are living organisms with a diameter of about 0.5 m. We can assert with complete confidence that there are no living organisms with a diameter of less than that of, let us say, an average-sided [sic] protein molecule. The point is, where within this range is the limit set? It is very probable that the size of the smallest living thing may be below that of the particles in a lytic filtrate, but I own to a feeling that we must be approaching the limit at which the inclusion of the necessary apparatus for nutrition and reproduction within a single cell will lead to uncomfortable crowding.Briefly to recapitulate the arguments which have been advanced.The lytic principle is an ultra-microscopic parasite, because it is particulate in nature, has the power of reproduction through an endless series of sub-cultures in symbiosis with a sensitive bacterium, and possesses a certain power of adaptation. It is not a living organism, because it can only increase in amount when the sensitive bacterium is actually dividing, a limitation which is not in accordance with most known facts of infection, because it can be precipitated by such agents as acetone, or aluminum hydroxide, and be recovered in an active form by solution in such substances as acetic acid or ammonia, and because its heat-resistance and persistent activity on prolonged storage suggest a chemical substance rather than a living organism. All the latter characteristics are, however, quite compatible with the active substance being a ferment. Moreover, its particulate nature is no evidence against this view, since there is not a priori reason why ferments should not be particulate, and every reason to believe, from analogy, that a ferment might readily be absorbed on to any particulate substance which was present in the filtrate. It is, however, almost impossible to believe that the substance is a ferment, since we should have to accept the view that it could only act on organisms which are actively dividing, having no effect on dead bacteria, or on living bacteria which were not undergoing multiplication. Moreover, a ferment cannot reproduce itself, so that we should have to believe that the organisms themselves produced more of the ferment, when they were undergoing destruction by it. A mechanism which reacts to a hurtful stimulus by promptly producing more of the harmful substance, and so leads straight to race suicide, does not seem likely to have led to prolonged survival, yet the bacteriophage and sensitive bacteria are widely distributed in nature. We can only conclude on a note of interrogation: What is the bacteriophage?........................................................................................................................................The Development of Plaques and the Mechanism of Phage Action in Solidified Agaras translated by Siobain Duffy and Stephen T. AbedonBacteriophage Ecology Group News (BEG News) 26Mayr-Harting, A. (1958). Die Entwicklung von Phagenloechern und der mechanismus der Phagenwirkung in festen Naehrboeden. Zentralblatt für Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene. 1. Abt., Originale (Zbl. f. Bakt. Paras. Infek. u. Hyg.) 171:380-392.From the Department of Bacteriology of Bristol University(Chairman: Prof. Dr. K. E. Cooper)The Development of Plaques and the Mechanism of Phage Action in Solidified AgarAnna Mayr-HartingWith 7 Figures in the TextReceived on 13 December 1957D' HERELLE occasionally reports, above all in his book “Le Bactériophage et son Comportement” (1926), that growing phage plaques on agar do not appear to change [in size] after they are visible to the eye. The plaques do not get bigger nor do the bacteria overrun them. This statement has never been challenged outside of a work by K. v. ANGERER (1924), which I sadly became aware after the following results were already completed.The isolation of a phage, which forms plaques that can potentially reach 12 mm in diameter allowed for research of plaque development. Already the first experiment clearly showed that plaques grow long after they become visible. The present study was undertaken in order to determine the general properties of this growth and the conditions that affect it.Agars and StrainsThe usual medium of this institute was used throughout: Ox-heart infusion; Bacto-Peptone 1%; NaCl 0.6%; Oxoid-Agar 2%; pH 7.6.The strain KS, which was used for amplification of the phage and in all of the experiments, was a typical bacterial coli from human feces.The phage strain came from a mixture of a large number of phages, that we use often for instruction purposes. This contained: 1. a mixture of coliphage from the Laboratory of Bacteriophage in Paris, that Dr. N. A. Boulgakov had kindly left, 2. “Enterofagos”, a mixture of phages for intestinal flora, from the company MedicoBiological Laboratories Ltd, London, 3. some phages from Bristol waste water. Despite frequent investigations of this phage mixture we had never noticed any large-plaque phage components. Also neither the strain KS itself nor any other strains of E. coli were recovered from any part of the phage mixture.The plaques show a concentric structure. The inner circle is completely sterile; a lightly clouded zone surrounds it; then follows a narrow, nearly sterile ring, and finally again a somewhat overgrown [region].The last [ring] shows a strange phenomenon that will be discussed shortly. During the first 24-30 hours it is not usually seen. In isolated plaques it only rudimentarily occurs. Where however plaques flow together, the ring occurs to a greater extent (Fig. 1). Merging plaques therefore were excluded from all measurements.ILLUSTRATION TO COMEFig 1. Direct photocopy of one of the plates with E. coli and phages. Synergistic effect of merging plaquesIf one lays a ring of the phage lysate on the bacteria-inoculated agar, that location appears perfectly sterile after incubation. A zone of partial lysis was already visible far into the bacterial lawns after 24 hours. This effect, as well as the effect of the merging plaques, extends over such distances that it was necessary to test if these outer zones were really components of a plaque or whether it was due to a lysin produced by the phages, in the sense of SERTIC (1929). Three strips of a bacterial lawn, of about a centimeter wide, were spread on agar plates; the remaining sterile gap between them was about 3 mm. Phage lysate was dripped on four points on the middle stripe. Although after 24 hours the cloudy zones of the plaques in the middle of the stripes were wider than the room between the stripes, there was no overgrowth [of the plaques] into the other stripes, and, after 48 hours, [the plaques overgrew] only in two places in the stripes, where, apparently, the bacteria from two stripes were confluent. It can therefore be assumed, that the partially cleared zones are a direct result of the phages, not of a soluble lysin (Fig. 2). ILLUSTRATION AT END OF ARTICLE Methods
The method employed was basically that of M. ADAMS (1952). Onto a 20ml agar Petri plate (9 cm diameter) was poured a 4ml top layer, which contained phages and bacteria. For the latter [top agar], 2 ml liquefied agar was mixed in a 50°C constant water bath with 1 ml of the bacterial culture or a resuspension and 1 ml of the appropriate phage dilution in broth. After solidifying, which occurred almost instantly, the plates were dried open for 15 minutes in the incubator, then closed and further incubated. The diameters of the plaques were measured with a caliper, through the glass, on the underside of the plate. The Nonius-scale of this instrument permits the reading [of measurements] of tenths of millimeters. The thickness of the glass and agar can distort the measurement by 0.1-0.2 mm. Results Fig. 3 shows the growth curves for three different plaques and three colonies of E. coli. The plaques were already visible after two to three hours. Their diameters grew linearly for 15-20 hours more. However, during this time the diameters of bacterial colonies grew exponentially. After transferring the data onto a logarithmic scale, the growth of the colony diameter for the first 10 hours had a constant slope and then the rate of increase became slower; however, some individual colonies grew with a constant slope all day. see Fig. 3.
In order to determine the environmental conditions that affect the growth and end size of plaques, quantitative research should be undertaken that does not focus on the growth of [an] individual plaque but [instead on] the average diameter of all of the non-overlapping plaques on the plate.
The environmental factors that were of importance were the thickness of the agar layer and the density of the bacterial inocula, and also, to a smaller degree, the concentration of the agar.
The age of the bacterial culture was completely irrelevant. Cultures from 6, 12, 24, and 48 hours gave plaques of the same size provided that the cultures were all diluted so that they all had the same number of living cells. Also, cold shock of the culture and cold shock followed by incubation had no influence on plaque growth.
The effect of placing poured phage plates in the refrigerator for 3 hours prior to incubation had the result of plaque growth lagging three hours behind the control culture. They eventually reached the same size.
Incubation at 22°C gave totally irregular results that cannot be explained at this time.
An experiment was conducted in salt-poor agar. The under layer was salt free. The over layer contained 1/40 or 1/80 of the normal 0.6%, respecitively. On the first plate the plaques were normal and in the latter the plaques were a bit smaller than in the controls.
D' HERELLE stated that the number of the plaques on a plate influenced the range of the sizes of the plaques in the same way as the number of bacterial colonies and their distance from each other influences the size of bacterial colonies. This cannot be said at all in the experiments presented here. Individual grown plaques have the same size without the ability to see the [other plaques in their] neighborhood. Completely in contrast to colonies of E. coli, which seldom overlap when they have reached a given size, but instead have a sterile space between them such that they remain independent, neighboring plaques grow over each other, which is an impressive synergistic phenomenon.
The agar concentration of the media, in combination with the variation in the incubation tested in the study, had a clear, but not very large effect. In Fig. 4 we showed that plaques became larger with lower concentrations of agar (in the top agar) but in one case [the plaques] were smaller on average [in lower-concentration agar] than in higher-concentration agar. In any case, the concentration of [agar] in the bottom agar layer had a clearer effect than that of the top agar. The plaques on the plate that had a bottom agar concentration of only 1% agar could only be seen up to 6 hours, and then the inoculum — the bacteria and phage — dissolved into an indefinite form. See Fig. 4. below The thickness of the lower agar layer during the first 6 hours affected plaque growth insignificantly. On the thinner layers, however, plaque growth ended sooner, while the thicker layers permitted longer enlargement of the plaque diameter (Fig. 5). The difference between the average diameters of the plaques on 10 ml and on 30 ml agar amounted to, after 45 hours, 5 mm and more.
|
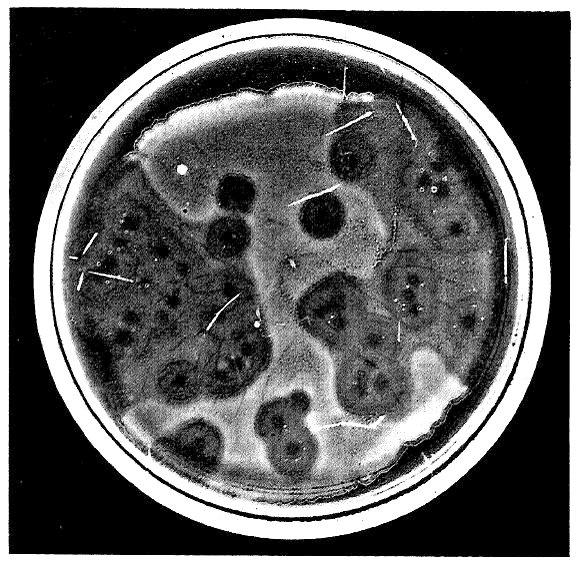 Fig 1. Direct photocopy of one of the plates with E. coli and phages. Synergistic effect of merging plaques. 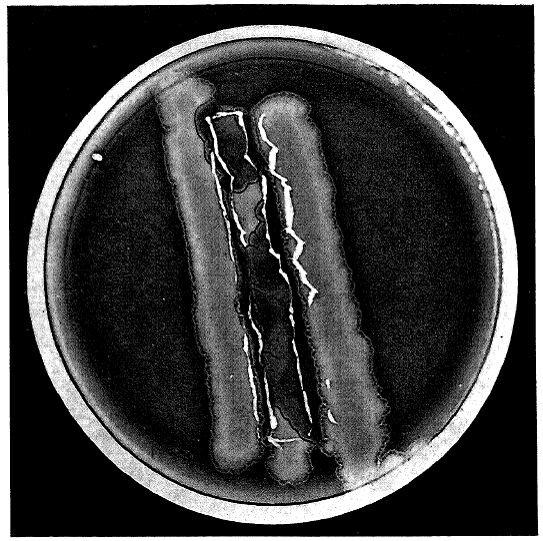 Fig. 2. Direct photocopy of one [plate] with three stripes of E. coli culture. Phage was dropped onto the middle striple at four points. Confluence of the phage between stripes only occured where the bacterial stripes grew into each other. At these [points], where the phages turned towards the sides of the inoculated stripes, [there] was a notable pearlescence of the edges, which was not very remarkable by eye, but showed up clearly in white in the photograph 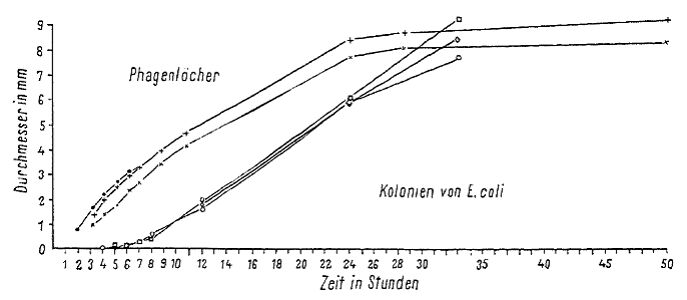 Fig. 3. Growth curves for the diameter of phages and E. coli colonies. The curve for the colonies was collected in two ways. For the first eight hours the colonies were observed microscopically and after eight hours [the growth of] three colonies that were the same size whether they were observed microscopically or macroscopically were followed on a plate. (translation from figure: x = time in hours, y axis = diameter in mm; plaques, colonies of E. coli) 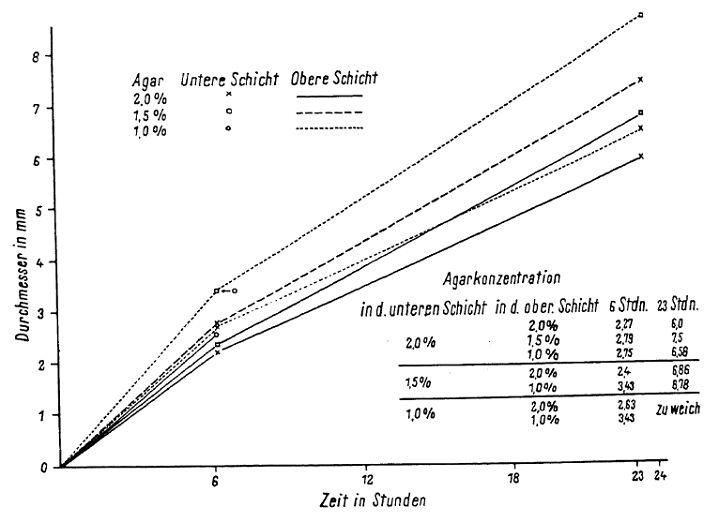 Fig. 4. Plaque size at different agar concen-trations. (x axis = time in hours, y = diameter in millimeters. Bottom layer agar concentration as symbols, top layer agar concentration as lines. Table: Agar concentration in the bottom layer (first column) and in the top layer (sec-ond column); diameter of the plaques after 6 hours (third column) and 23 hours (fourth column). “Zu weich” – too soft) 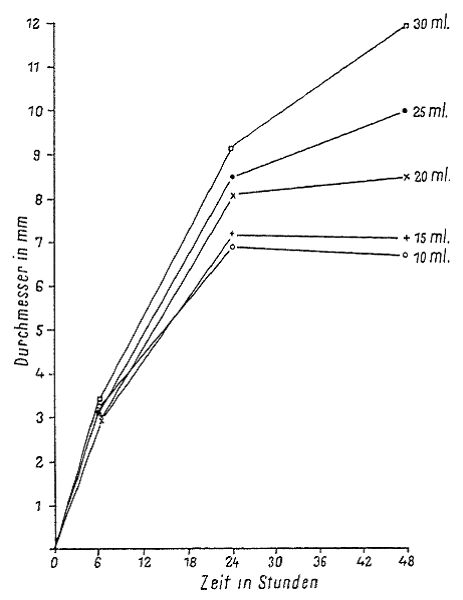 Fig. 5. Growth of plaques with differently thick bottom agar layers. (x axis = time in hours , y = diameter in millimeters) |